Background: New therapeutic strategies are strongly needed to improve the prognosis of high risk Acute Myeloid Leukemia (AML) patients, such as combination of novel agents and conventional chemotherapy. The Italian GIMEMA AML1718 trial (NCT03455504) investigate the safety and efficacy of the BCL-2 inhibitor venetoclax (VEN) in combination with intensive fludarabine-based induction (FLAI) [fludarabine 30 mg/sqm from day 1 to day 5, cytarabine 2000 mg/sqm from day 1 to day 5, and idarubicin 8 mg/sqm on days 1, 3 and 5] as first-line therapy for newly diagnosed non low-risk ELN AML patients. Results from the Planned Interim Analysis of Safety Run-in and Part 1 (early expansion cohorts) were presented at the last 2022 ASH Meeting. Median overall survival (OS) was not reached; probability of 12-month OS was 76%. Median disease-free survival was not reached. With a median follow-up of 10.5 months, 28 patients (49%) received allogeneic stem cell transplantation (HSCT) in first complete remission (CR).
Centralized multicolour flow cytometry minimal residual disease (MFC-MRD) assessment was planned during the phase 2, part 2 of the study (confirmatory cohort) where the lower effective dose of VEN (400 mg/day) was administered in association with FLAI. Here we report results from early time-points (TPs) MRD analysis with the aim of identify the most informative TPs for MRD assessment and prognostic correlations.
Methods: Erythrocyte-lysed whole bone marrow (BM) samples obtained at diagnosis from patients enrolled in the confirmatory cohort were centralized and analysed with a broad panel of monoclonal antibodies to identify the leukemia-associated phenotype (LAIP) which was used to track residual leukemic cells during follow-up. Eight color flow cytometry analysis was performed at pre-defined TPs (TP1: post-induction I, TP2: post induction II/consolidation I, following TPs: post consolidation/pre-transplantation) (FACSCantoII; BD Facs Diva Software V6.1.3). A positive flow MRD was defined by the presence of no less than 10 clustered leukemic cells/10^4 total events. Enrollment closed on January 2023. Collection and analysis of later TPs is ongoing.
Results: Sixty-seven patients from 11 centers were enrolled in the phase 2, part 2. Risk stratification according to ELN 2017 was intermediate in 46% of patients and high risk in 54%. Fifty-eight/67 patients (87%) obtained CR after induction I.
In the centralized MRD analysis, 170 samples has been collected and analysed so far (60 baseline samples, 110 MRD samples). Seven patients lacking baseline sample for LAIP identification were excluded from the analysis. TP1 was available in 57/59 patient achieving CR (97%), and TP2 was available in 29 patients, so far.
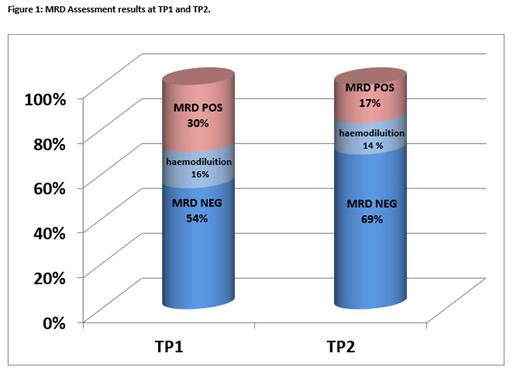
MFC-MRD negativity was obtained in 31/57 (54%) at TP1 (post V-FLAI, Fig. 1). An increase in MFC-MRD negativity rate was observed at TP2 (post Induction II or consolidation I) with 20 MFC-MRD negative patients/29 available samples (69%, Fig. 1).
Thirteen/110 follow up samples (8%), albeit resulting MRD negative, were considered inadequate for the analysis due to haemodilution, 9 from TP1 and 4 from TP2 (Fig. 1).
TP1 and TP2 MRD assessment were scheduled at day 28 of Induction I and Induction II/consolidation, respectively. However, in most cases, delayed haematological recovery was observed after V-FLAI, thus resulting in suboptimal samples for MRD evaluation.
Conclusions: Preliminary results from centralized MRD analysis confirm that the combination therapy is able to induce high-quality remissions with a very high percentage of MFC-MRD negativity in a difficult cohort of patients, with a higher percentage of MFC-MRD-negative after the completion of the second course of therapy. Delayed haematological recovery may impact on reliability of MRD assessment due to hypocellular and regenerative marrow samples, suggesting that in those cases MRD analysis should be postponed. Correlation with survival will be performed as the data collection will be complete.
OffLabel Disclosure:
Papayannidis:Abbvie, Astellas, Servier, Menarini/Stemline, BMS, Pfizer, Amgen, Janssen, Incyte, Novartis: Honoraria; Pfizer, Astellas, Janssen, GSK, Blueprint, Jazz Pharmaceuticals, Abbvie, Novartis, Delbert Laboratoires: Membership on an entity's Board of Directors or advisory committees. Bocchia:BMS: Honoraria; Incyte: Honoraria; Novartis: Honoraria. Della Porta:Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees. Frigeni:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceutical: Honoraria. Borlenghi:AbbVie, BMS: Consultancy; Amgen, Incyte: Other: travel grants. Venditti:Amgen: Consultancy, Honoraria, Other: travel support ; Pfizer: Consultancy, Honoraria, Other: travel support , Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: travel support ; Medac: Consultancy; Novartis: Consultancy, Honoraria, Other: travel support ; Janssen: Consultancy, Honoraria, Other: travel support ; Jazz: Consultancy, Honoraria, Other: travel support . Vignetti:Novartis: Speakers Bureau; Dephaforum: Honoraria; AbbVie: Honoraria; Uvet: Honoraria; IQVIA: Honoraria; ER Congressi: Honoraria.
Venetoclax added to chemotherapy in newly diagnosed AML